RAG-01: Clinical Phase I
RAG-01:
Indication: Non-muscle invasive bladder cancer (NMIBC)
Non-muscle invasive bladder cancer (NMIBC) constitutes 70-80% of all bladder cancer cases. Typical treatments include transurethral resection of the bladder tumor (TURBT) followed by repeated intravesical instillations of chemotherapy drugs or Bacillus Calmette-Guérin (BCG).
Despite these interventions, the 5-year recurrence rate remains high at 50-70%, underscoring a substantial unmet need for more effective and less burdensome treatment
A primary driver of tumorigenesis in NMIBC is the loss of tumor suppressor genes, particularly through disruptions in the p53-p21WAF1/CIP1 (p21) pathway. Addressing this pathway presents a promising approach to cancer therapy. RAG-01, a novel saRNA therapy developed by Ractigen Therapeutics, targets and activates the traditionally “undruggable p21 gene through the RNAa mechanism. By reactivating this key regulatory pathway, RAG-01 aims to prevent recurrence and impede progression of NMIBC. Promising results from preclinical studies have demonstrated that RAG-01 significantly suppresses tumor growth in mouse orthotopic bladder cancer models.
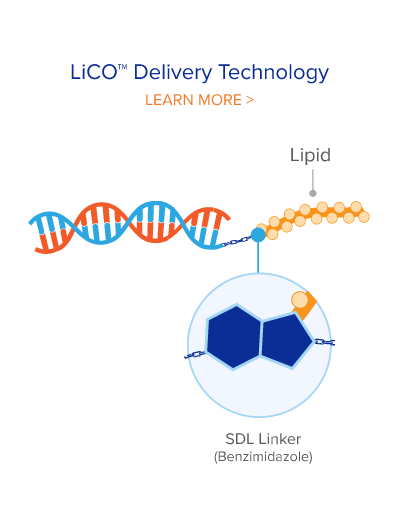
Administered through intravesical instillation, RAG-01 utilizes Ractigen’s proprietary LiCO™ delivery technology, which efficiently penetrates the glycosaminoglycan layer lining the urothelium and delivers the saRNA directly to the urothelial cells. This localized delivery method allows high drug concentration in the bladder urothelium while minimizing systemic exposure and potential side effects.
RAG-01 is currently in an open-label, dose-escalation, multi-center Phase I study to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, and preliminary efficacy of RAG-01 in NMIBC patients unresponsive to BCG therapy.

RAG-17: Clinical Phase I
RAG-17: Program News
Indication: Amyotrophic Lateral Sclerosis (ALS)
Amyotrophic Lateral Sclerosis (ALS), often known as Lou Gehrig’s disease, is a devastating neurodegenerative disorder that gradually destroys motor neurons in the brain and spinal cord. A subset of this disease, SOD1-ALS, is caused by mutations in the SOD1 gene, which account for about 20% of all familial ALS cases and 1-2% of sporadic ALS cases. These mutations lead to the production of a toxic protein that contributes to the degeneration of motor neurons. The average life expectancy after diagnosis is only 2-5 years, and current treatments can only modestly extend survival or slow the progression. Thus, there remains a significant unmet medical need for more effective therapies.
Ractigen Therapeutics is advancing RAG-17, a pioneering siRNA therapy designed to silence the SOD1 gene and reduce the production of the destructive protein implicated in SOD1-ALS. Unlike traditional drugs, RAG-17 utilizes the power of RNA interference (RNAi) to act at the root cause of the disease. This therapeutic agent utilizes Ractigen’s innovative SCADTM architecture, which links the duplex siRNA to a short oligonucleotide aptamer, facilitating efficient and sustained delivery within the central nervous system (CNS) via intrathecal injection.
Extensive preclinical studies in mouse and rat models of SOD1-ALS have demonstrated the remarkable efficacy of RAG-17. It significantly delayed the onset of ALS symptoms, enhanced motor functions, and extended survival. Remarkably, RAG-17 remained effective even when administered after disease onset, offering hope for patients already experiencing the devastating effects of ALS. These outcomes suggest that RAG-17 could dramatically alter the treatment paradigm for patients with SOD1-ALS, providing them with the prospect of an improved quality of life and extended longevity.
An ongoing Investigator Initiated Trial (IIT) is currently assessing the safety, tolerability, and pharmacokinetics of RAG-17 in adult ALS patients with an SOD1 mutation.
RAG-17: Program News
Indication: Amyotrophic Lateral Sclerosis (ALS)
RAG-17: A Novel siRNA Conjugate Demonstrating Efficacy in Late-Stage Treatment of SOD1G93A ALS mice
Duan C, Kang M, Liu K, Gan Z, Li G, Chen J, Schacht I, Place RF, Li LC. RAG-17: A Novel siRNA Conjugate Demonstrating Efficacy in Late-Stage Treatment of SOD1G93A ALS mice. bioRxiv. 2023:2023-11. DOI: 10.1101/2023.11.23.568255
Intrathecal administration of a novel siRNA modality extends survival and improves motor function in the SOD1G93A ALS mouse model
Duan C, Kang M, Pan X, Gan Z, Huang V, Li G, Place RF, Li LC. Intrathecal administration of a novel siRNA modality extends survival and improves motor function in the SOD1G93A ALS mouse model. Molecular Therapy-Nucleic Acids. 2024 Mar 12;35(1). DOI: 10.1016/j.omtn.2024.102147. PMID: 38435120; PMCID: PMC10907209.